1. Preface
With the continuous promotion of national strategies such as energy conservation and emission reduction, the flue gas denitrification technology of coal-fired power plants has gradually matured. The proportion of non-electric power industries in atmospheric treatment is gradually increasing, and some of them emit flue gas with low temperature, such as coking, cement, glass, industrial boilers, waste incineration, etc. Therefore, the research of efficient low-temperature denitrification technology is an important direction of the current denitrification process. The current commercial catalysts are mainly V2O5-WO3, MoO3 /TiO2, with TiO2 as the carrier, V2O5 as the active component and WO3 or MoO3 as the active additive. The addition of the active additive improves the high and low temperature activity of the catalyst and effectively inhibits the occurrence of side reactions. However, the catalyst is a medium-high temperature catalyst with an active temperature window of 300-400°C. Below or above this temperature range, the denitrification activity of the catalyst starts to decline and reversible/irreversible poisoning deactivation occurs, which cannot meet the needs of industries with flue gas emission temperatures below 300°C. If the flue gas reheating by denitrification process is used, it will lead to increased energy consumption. Using low-temperature SCR denitrification can place the denitrification process after the dust removal or desulfurization process to reduce the wear and poisoning effect of soot on the catalyst, avoid flue gas reheating, and thus improve energy efficiency and save operating costs. Therefore, it is very important to study the performance of efficient low temperature denitrification catalysts for the low temperature denitrification industry.
2.Low temperature catalyst R&D direction
Difficulties of low-temperature denitrification catalysts.
(1) Low denitrification activity: denitrification catalyst activity generally decreases as the flue gas temperature decreases, and when the temperature is below 200℃, the existing low temperature catalyst activity is low, which will cause the denitrification efficiency to be substandard, and also lead to secondary pollution problems such as ammonia escape over the standard;
(2) Poor anti-sulfur poisoning performance: SO2 and SO3 in the flue gas will react with the active bits of the catalyst, resulting in the reduction of the number of active bits and the degradation of denitrification performance.
(3) Serious blockage poisoning: SO2 in flue gas formed by oxidation of SO3 will react with NH3 to produce sulfur ammonium salts, which will adhere to the catalyst surface, causing the catalyst active site to be covered, and the sulfur ammonium salts will further adsorb fly ash in the flue gas, aggravating the blockage and leading to rapid catalyst deactivation.
(4) Poor water vapor tolerance: During low-temperature SCR denitrification, the water vapor present in the flue gas can affect the denitrification efficiency of the catalyst through physical competitive adsorption and chemisorption interference reactions.
Low temperature SCR denitrification catalysts are still in the research stage in terms of selective catalysis, service life, performance stability and catalytic effect. During the research, SO2 and water vapor have certain toxic effects on the catalysts, and the SO2 and water vapor tolerance of the catalysts can be improved by improving the catalyst preparation methods and selecting suitable catalyst active ingredients and carriers. Therefore, in-depth research is needed to carry out the low-temperature SCR denitrification catalysts with respect to water vapor and SO2 resistance.
3. Progress of low-temperature catalyst research by the team of Environmental Protection Technology Center of Beijing Low Carbon Clean Energy Research Institute (Low Carbon Institute) of National Energy Group
Low Carbon Institute researchers firstly discovered the acidic effect of molecular sieve on the redox performance of the active component, and revealed the mechanism of low-temperature reaction involving the "double active center" of active and acidic sites, which was published in the Nature subjournal Communications Chemistry. The results of this study were published in the Nature subjournal Communications Chemistry.
Currently, more than 60% of coal-fired units in China are at low load, and the flue gas temperature is often below 300°C, when the denitrification activity of V-W-Ti catalysts is poor. Therefore, the development of denitrification catalysts with high activity at low temperature (<300℃) is important to support coal-fired power plants to achieve full-load denitrification with deep peaking of new energy. Also low-temperature denitrification technology is in great demand for flue gas cleaning in non-electricity fields.
Manganese oxides are a commonly used active component of low temperature denitrification catalysts. It is generally believed that MnO2 has high low-temperature denitrification activity, while Mn2O3 has the best N2 selectivity. How to balance the denitrification activity and selectivity at the same time becomes the biggest challenge for the molecular design of manganese based denitrification catalysts. Meanwhile, pure silicon mesoporous molecular sieve carrier with high specific surface area and rich pore structure limits its application in denitrification catalysis due to low acidity.
In response to these problems, researchers from the denitrification team of the Low Carbon Institute, in collaboration with researchers from the Clean Environment and Energy Center of Griffith University, Australia, have conducted calculations on the thermodynamics of the active components of the catalysts based on density flooding theory (DFT) and in situ infrared characterization, and investigated the reactant adsorption process on the catalyst surface by using the molecular simulation software VASP, and found for the first time that the acidity of molecular sieves has a significant effect on the active The mechanism of low-temperature reaction involving the "dual active centers" of active and acidic sites was also revealed.
Guided by this reaction mechanism, the researchers used Si and Al elements in coal-based solid waste fly ash to controllably synthesize Al-SBA-15 mesoporous molecular sieves with different skeletal Si/Al ratios, and the results of Py-IR combined with various NMR analyses showed that the doping of Al significantly improved the acidity of the molecular sieve; and the synergistic effect of L and B acids effectively regulated the growth of the active component MnO The most suitable ratio of MnO2 and Mn2O3 content was obtained.
It was found that the introduction of Al not only induced the crystalline transformation of the active component MnOx, but also induced its grain size and crystal growth position by XRD, XPS, NH3-TPD, HAADF-STEM and other analytical test characterization methods. The experimental results showed that the fugitive state of manganese in Fe-Mn/Al-SBA-15 catalyst was more favorable for the NH3-SCR reaction.
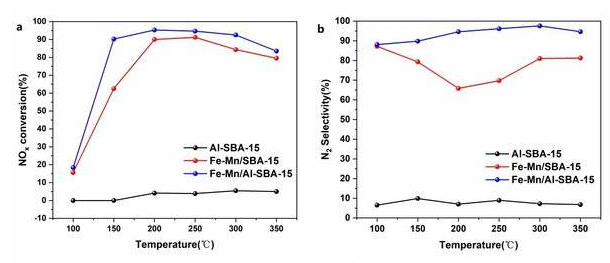
The NH3-SCR denitrification activity of the catalysts was tested, and it was found that the denitrification catalysts prepared by Low Carbon Institute had both high NOx conversion (≥90%) and good selectivity (≥86%) at low temperatures (150-300°C).

